Reações em que uma transferência de elétrons ocorre, as chamadas reações redox ou de oxirredução, são uma parte importante para a compreensão de processos eletroquímicos, como os que ocorrem na corrosão de metais e em pilhas e baterias.
É comum se realizar experimentos com o sulfato de cobre e palha de aço, por exemplo. Nesses experimentos, embora seja muito fácil se observar a formação de um depósito de cobre metálico, não é tão fácil se perceber que a palha de aço está sendo oxidada e o ferro indo para a solução.
Neste experimento podemos ver com facilidade a redução dos íons de cobre e a oxidação do alumínio metálico. E com um gasto mínimo de reagentes, formação de resíduos também mínima e com uma limpeza muito simples. Vamos cortar uma lata de alumínio somente com a Química.
Corte a lata e desenhe com um estêncil


Precisamos deixar a parte da lata com o desenho plana. Para isso, dobramos as quatro pontas do quadrado para cima.
Em seguida, colocamos o quadrado de lata sobre uma pasta de plástico em L com uma folha de papel A4 dentro. A pasta é só para se ter uma superfície de trabalho fácil de limpar e para dar um bom contraste para a cor da solução após a reação.
Adicionamos sobre a parte da lata que foi raspada algumas gotas (em torno de 5) de uma solução de cloreto de cobre 1 mol/L. Caso você não tenha cloreto de cobre, você pode usar sulfato de cobre a acrescentar um pouco de cloreto de sódio à solução.

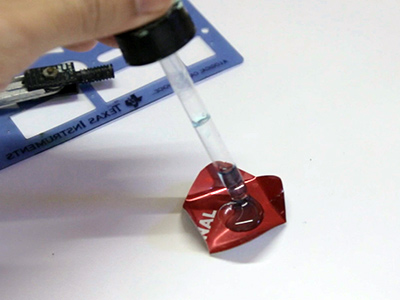
Observe, filme e fotografe
Agora é hora de observar a reação. Para observar bem de perto, e registrar o que está acontecendo, a sugestão é usar a câmera do celular dos alunos. Providencie um apoio para o celular de modo que ele possa enquadrar bem a reação.
Em segundos é possível observar a formação de um depósito escuro sobre a parte exposta do alumínio. Também podemos observar a liberação de bolhas de um gás.
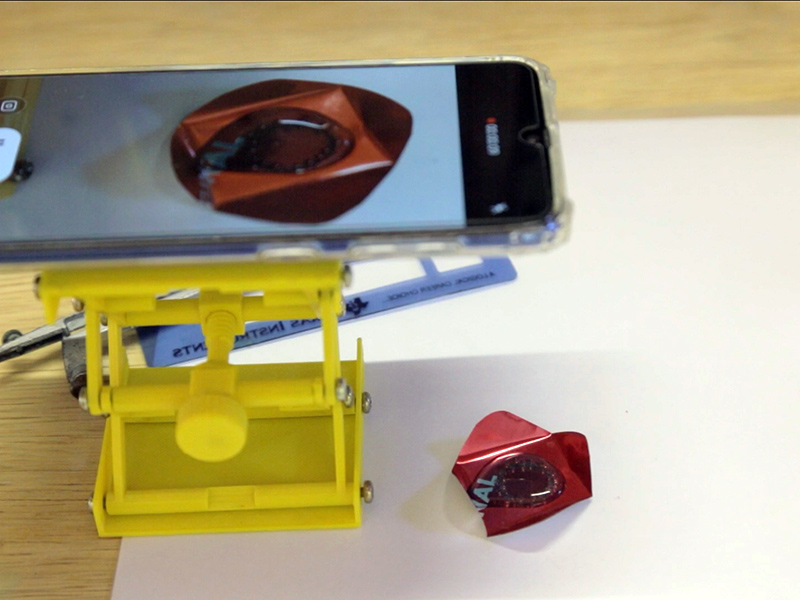
Continue acompanhando a reação até que a liberação de gás tenha diminuído muito. Você pode sentir com a ponta do compasso ou alfinete se o alumínio ao redor da figura já foi completamente dissolvido e se é possível levantar uma ponta e retirar o círculo de alumínio do centro. Isso leva em torno de 2-3 minutos.



Você pode criar um estêncil com as suas formas prediletas usando a impressão 3D. É uma ótima maneira de se envolver os alunos e eles aprenderem a usar o TinkerCAD. E se você não tiver um compasso pode usar um alfinete.

O que aconteceu?
Temos várias evidências de que uma reação química aconteceu. A solução azul contendo íons de cobre 2+ se tornou incolor. Um sólido de cor marrom acastanhado se formou sobre a parte do alumínio que ficou exposta. Bolhas de um gás apareceram durante a reação. E, talvez o mais impressionante, nós conseguimos cortar a lata de alumínio.
Os íons de cobre 2+ dão a cor azul à solução inicial. Vemos que essa cor desaparece e que aparece um depósito marrom sobre a área de alumínio exposta pela agulha do compasso. Podemos então escrever uma equação em que temos os íons de cobre como reagentes e o cobre como produto.

Os íons de cobre estão recebendo elétrons e se reduzindo para cobre metálico. A equação fica assim:
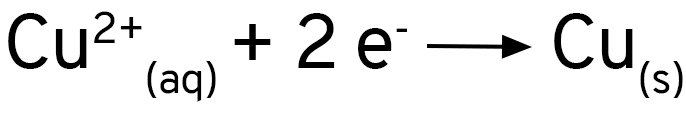
Se você adicionar uma solução de sulfato de cobre a uma placa de alumínio, verá que nada acontece. Ao se usar cloreto de cobre, ou se adicionar um pouco de cloreto de sódio ao sulfato de cobre, verá que a reação acontece imediatamente. O alumínio forma uma camada de óxido sobre a sua superfície, que protege o metal contra a oxidação. Os íons cloreto removem essa camada e expõe o alumínio ao ataque dos íons de cobre.
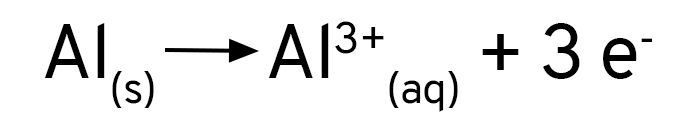
Mas, de onde vêm as bolhas que nós vimos? Que gás é esse? Quando os íons cloreto expõe a superfície do alumínio, ele não reage apenas com os íons de cobre em solução. O alumínio é tão reativo que ele reage também com a água! Os íons de hidrogênio são reduzidos pelo alumínio e formam o gás hidrogênio.
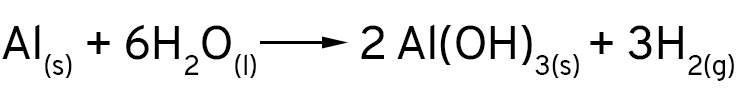
Finalmentes
Este é um ótimo exemplo de reação em microescala, onde conseguimos ao mesmo tempo diminuir as quantidades, diminuir o risco e o impacto ambiental e ao mesmo tempo possibilitar uma melhor compreensão sobre o fenômeno.

